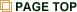Department of Applied Chemistry, Graduate School of Engineering, Osaka University
Research
Hemoprotein Engineering
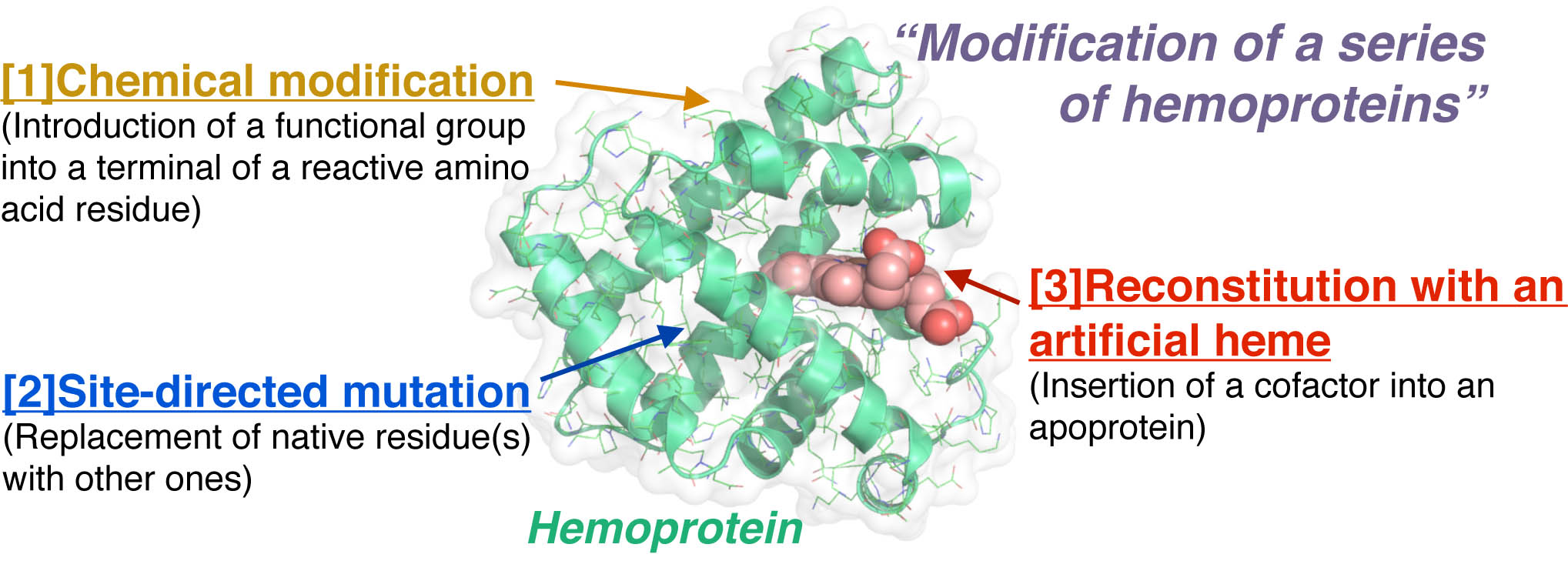
Hemoproteins, which have iron porphpyrins as a prosthetic group, show a variety of functions derived from the unique combination of the heme and protein matrix. Thus, it is of considerable interest to engineer the function of the hemoproteins. To modify the hemoproteins, at least there are three methods as the following figure. Our group mainly focuses on the third method, “Insertion of an artificially created heme into an apoprotein” to yield a reconstituted protein to understand the function and/or enhance the reactivity.1
Enhancement of Hemoprotein Reactivity
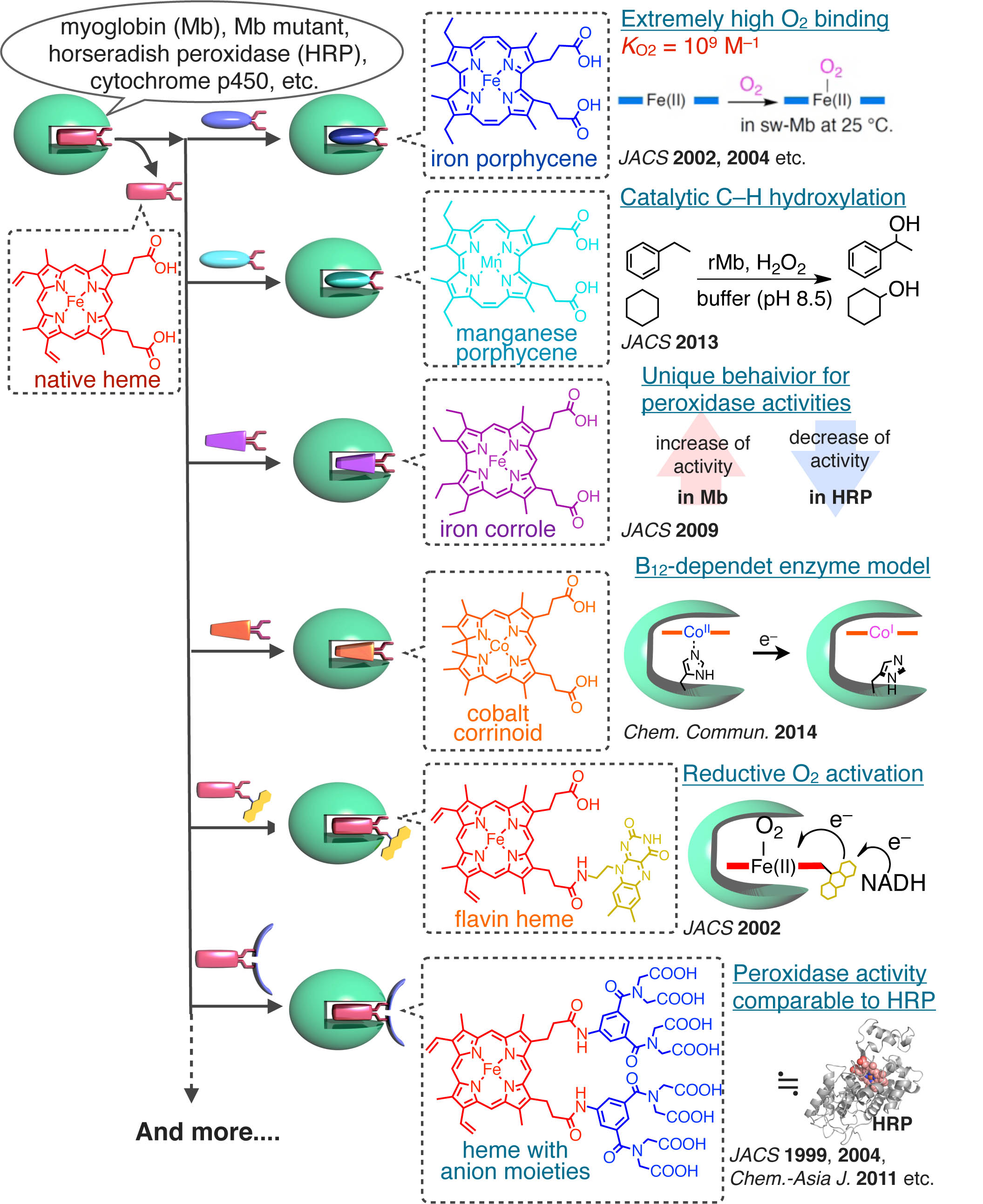
Heme b (protoporphyrin IX iron complex) which is bound in the heme pocket via multiple non-covalent interactions is the prosthetic group most often seen in a series of hemoproteins. To enhance and/or modify the hemoprotein functions, we have prepared variouis functionalized artificial heme derivatives or analogs (isomers) and replaced the native heme with them as shown in the following scheme. The obtained reconstituted hemoproteins exhibit unique physicochemical or enzymatic properties: i) extremely high O2 affinity,2-5 ii) remarkable peroxidase/peroxygenase/hydroxylase activity,6-12 iii) reductive O2 activation,13 iv) interprotein electron transfer and v) B12-dependent enzyme model.14 Target proteins for our research are myoglobin, hemoglobin, horseradish peroxidase, cytochrome b562, cytochrome c, cytochrome P450cam, heme oxygenase etc. and those mutants.
- 2) J. Am. Chem. Soc., 124, 11226-11227 (2002).
- 3) J. Am. Chem. Soc.,126, 16007-16017 (2004).
- 4) J. Am. Chem. Soc.,127, 56-57 (2005).
- 5) Inorg. Chem.,44, 9391-9396 (2005).
- 6) J. Am. Chem. Soc.,121, 7747-7750 (1999).
- 7) J. Am. Chem. Soc.,126, 436-437 (2004).
- 8) Inorg. Chem., 45, 10530-10536 (2006).
- 9) J. Am. Chem. Soc.,129, 12906-12907 (2007).
- 10) J. Am. Chem. Soc., 131, 15124–15125 (2009).
- 11) Chem. Asian J., 6, 2491–2499 (2011).
- 12) J. Am. Chem. Soc., 135, 17282–17285 (2013).
- 13) J. Am. Chem. Soc., 124, 11234–11235 (2002).
- 14) Chem. Commun., 50, 12560–12563 (2014).
Molecular Recognition on the Protein Surface
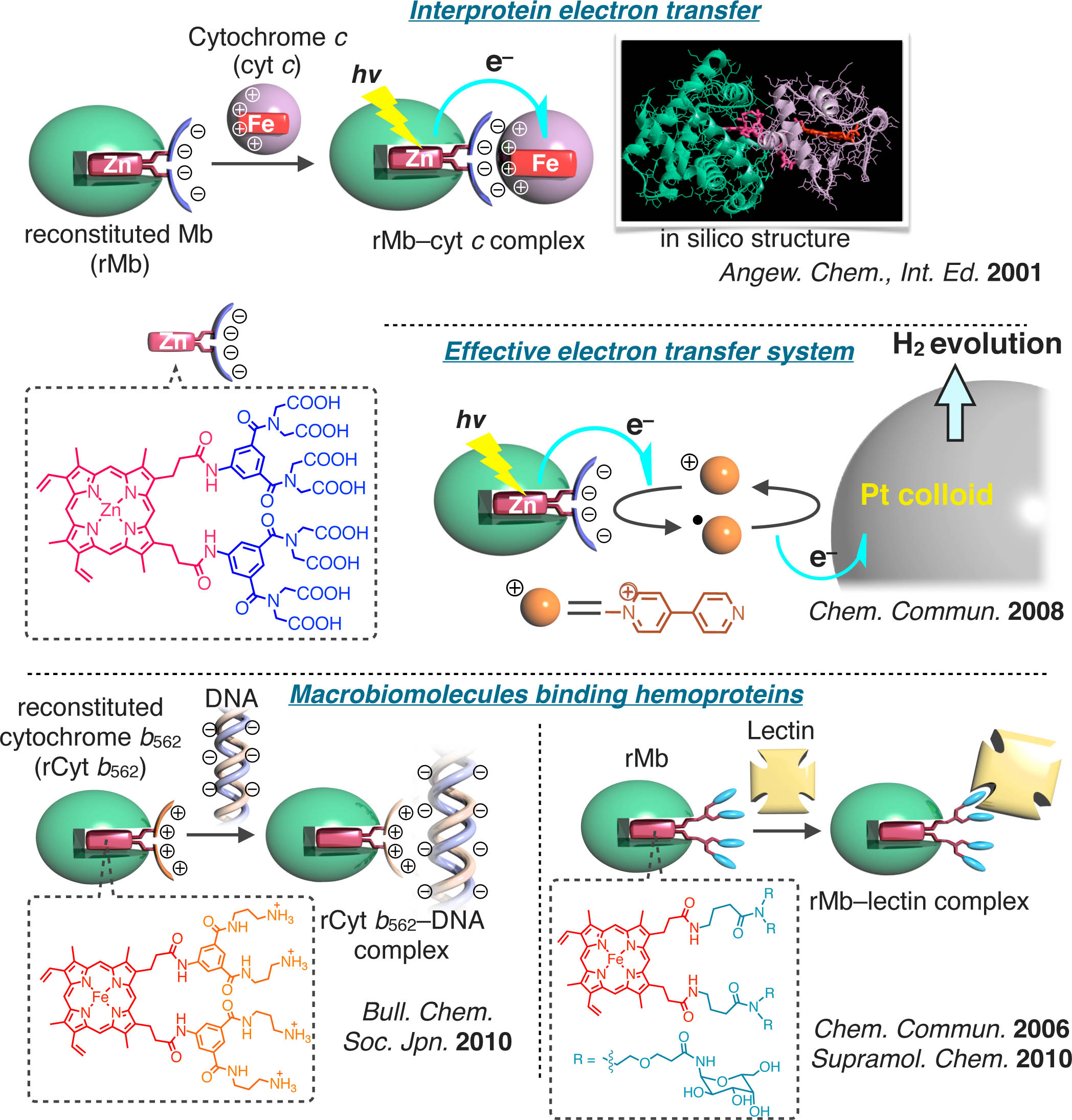
Biological communication on the protein or cell surface is one of the attractive phenomena. Thus, it is of particular interest to understand the physicochemical properties of protein–protein interaction. Our group has proposed that the modification of hemoprotein with artificial heme which has multiple functional groups such as carboxylate, ammonium or carbohydrate is effective for generating new interprotein complexes: i) myoglobin with an anionic patch on the protein surface to make a complex with a positively charged cytochrome c,15,16 ii) the complex between negatively charged zinc myoglobin and cytchrome c toward the model for an interprotein electron transfer in mitochondrial respiratory system,17 iii) multiple electron transfer system of zinc myoglobin with the anionic patch with positively charged methyl viologen and Pt colloid toward efficient H2 gas generation,18 iv) DNA binding cytochrome b562 with polyamine interface,19 v) myoglobin with sugar moieties to complex with a sugar binding protein such as peanut lectin.20,21
- 15) Chem. Soc. Rev., 126, 355–364 (1997).
- 16) J. Am. Chem. Soc., 120, 4910–4915 (1998).
- 17) Angew. Chem., Int. Ed., 113, 1132–1135 (2001).
- 18) Chem. Commun., 31, 3684–3686 (2008).
- 19) Bull. Chem. Soc. Jpn., 4, 375–377 (2010).
- 20) Chem. Commun., 29, 3131–3133 (2006).
- 21) Supramol. Chem., 22, 57–64 (2010).
Supramolecular Protein Assembly and Nanoparticle Conjugate
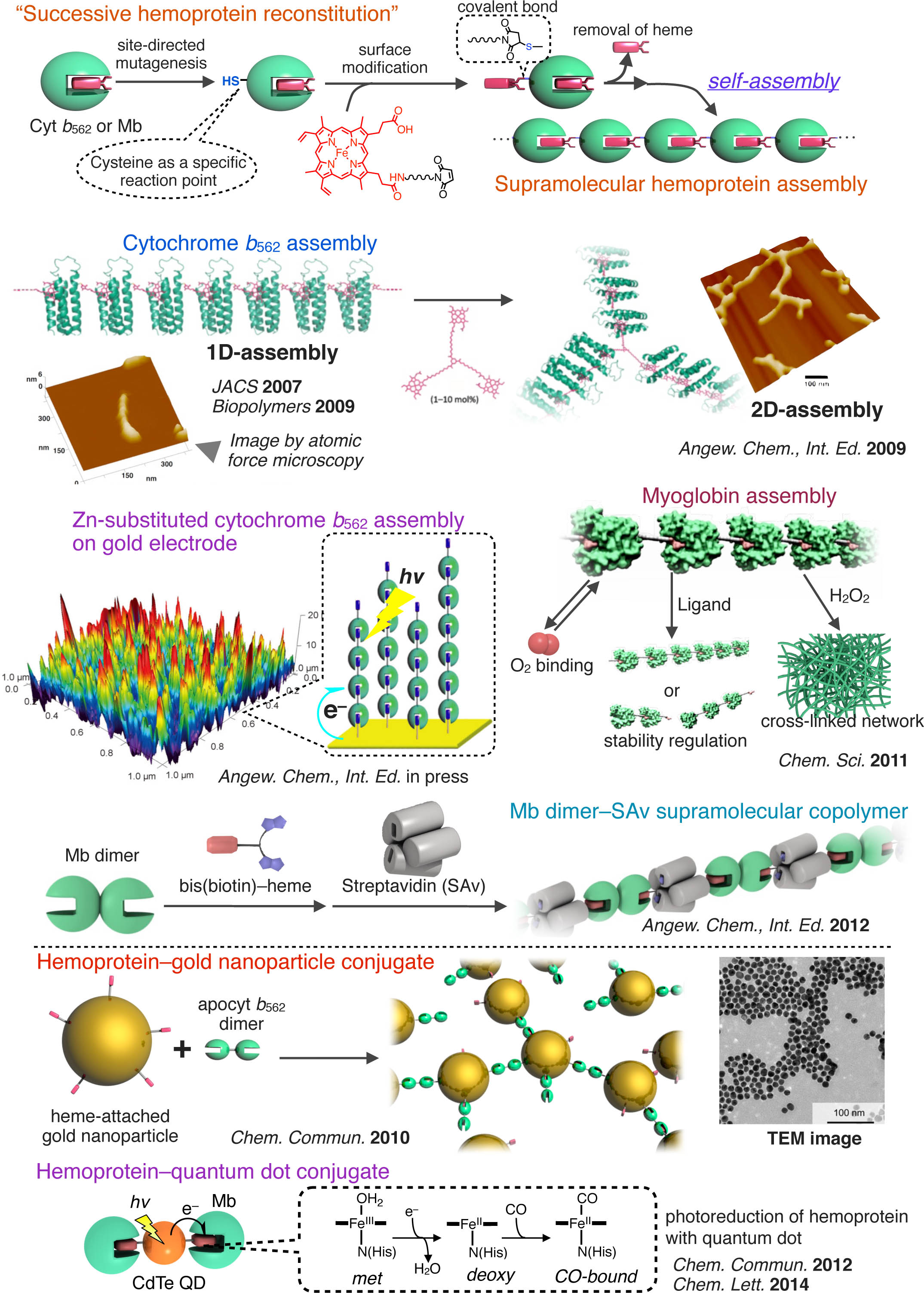
Modifying the architecture of a supramolecular polymer is a promising tactic to mimic a biological system and construct bionanomaterials. We found that the heme–heme pocket interaction with an affinity of 1010 – 1015 M-1 serves as a new way to prepare an attractive supramolecular hemoprotein polymer or hemoprotein–nanoparticle conjugates22 as follows; i) 1D self-assembling system with heme-attached cytochrome b562 mutant to afford the sub-micrometer sized fibrous assembly,23,24 ii) 2D hemoprotein assembly network derived from 1D self-assembling system and a synthetic heme triad,25 iii) functional hemoprotein assembling system using myoglobin, an oxygen storage protein, as a monomer,26 iv) zinc substituted cytochrome b562 assembly immobilized on a gold electrode to achieve efficient photocurrent generation,27,28,29 v) the supramolecular heterotropic assemblies such as co-assembly of hemoprotein and streptavidin with alternating alignment via the (bis)biotin-heme conjugate,30, 31 vi) metal nanoparticle–hemoprotein assemblies. 32, 33, 34
- 22) Chem. Commun., 48, 11714–11726 (2012). Feature Article
- 23) J. Am. Chem. Soc., 129, 10326–10327 (2007).
- 24) Biopolymers, 3, 194–200, (2009). Front Cover
- 25) Angew. Chem., Int. Ed., 48, 1271–1274 (2009). Inside Front Cover
- 26) Chem. Sci., 2, 1033–1038 (2011). Backside Cover
- 27) Angew. Chem., Int. Ed., 512628–2631 (2012). Hot Article
- 28) J. Inorg. Organomet. Polym. Mat., 23, 172–179 (2013).
- 29) Dalton Trans., 42, 16102–16107 (2013).
- 30) Angew. Chem., Int. Ed., 51, 3818–3821 (2012). Front Cover
- 31) Chem. Biodivers., 9, 1684–1692 (2012).
- 32) Chem. Commun., 9107–9109 (2010). Front Cover
- 33) Chem. Commun., 48, 8054–8056 (2012). Inside Front Cover
- 34) Chem. Lett., 43, 1152–1154 (2014).
Construction of Di- or Multi Metal Center in Protein Matrix
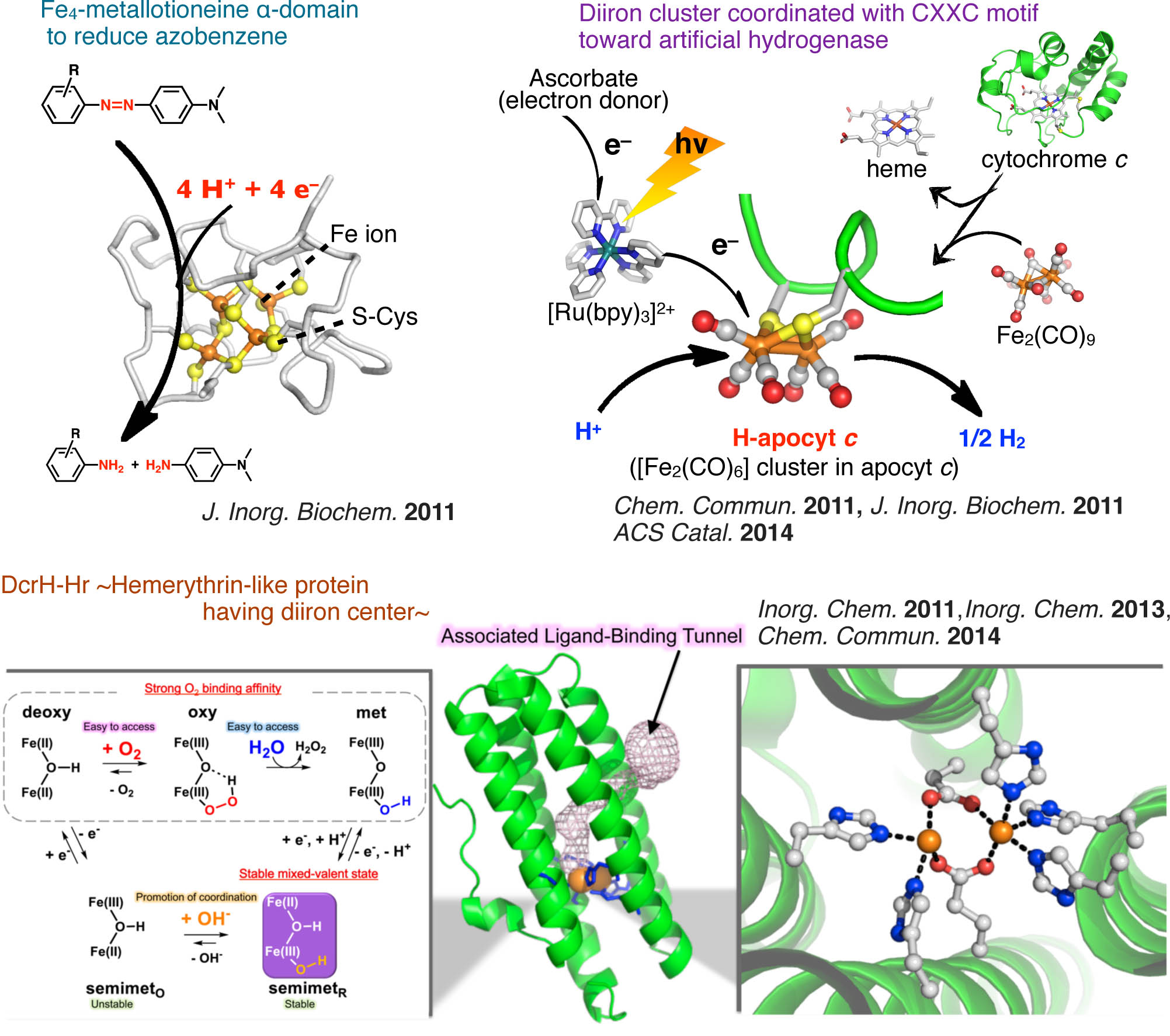
Attractive biological reactions catalyzed by enzymes with multi-metal center (ex. nitrogenase, hydrogenase etc.) have been noted due to their potentials to solve the energy and environmental problems. However, these enzymes are generally large and extremely complicated. In this context, we have challenged to investigate both of native and artificial multi metal center in small protein matrices toward such enzymatic reactions: i) reduction of azobenzene derivatives by metallothionein-iron(II) complex,35 ii) hydrogen evolution by diiron carbonyl cluster in apocytchrome c or small peptide containing CXXC motif as a artificial hydrogenase model,36,37,38 iii) detailed evaluation for mixed-valent oxidation state of DcrH-Hr, hemerythrin-like protein with diiron center.39,40,41
- 35) J. Inorg. Biochem., 702–708 (2011).
- 36) Chem. Commun., 8229–8231 (2011). Inside Front cover
- 37) J. Inorg. Biochem., 108, 159–162 (2012).
- 38) ACS Catal., 4, 2645–2648 (2014).
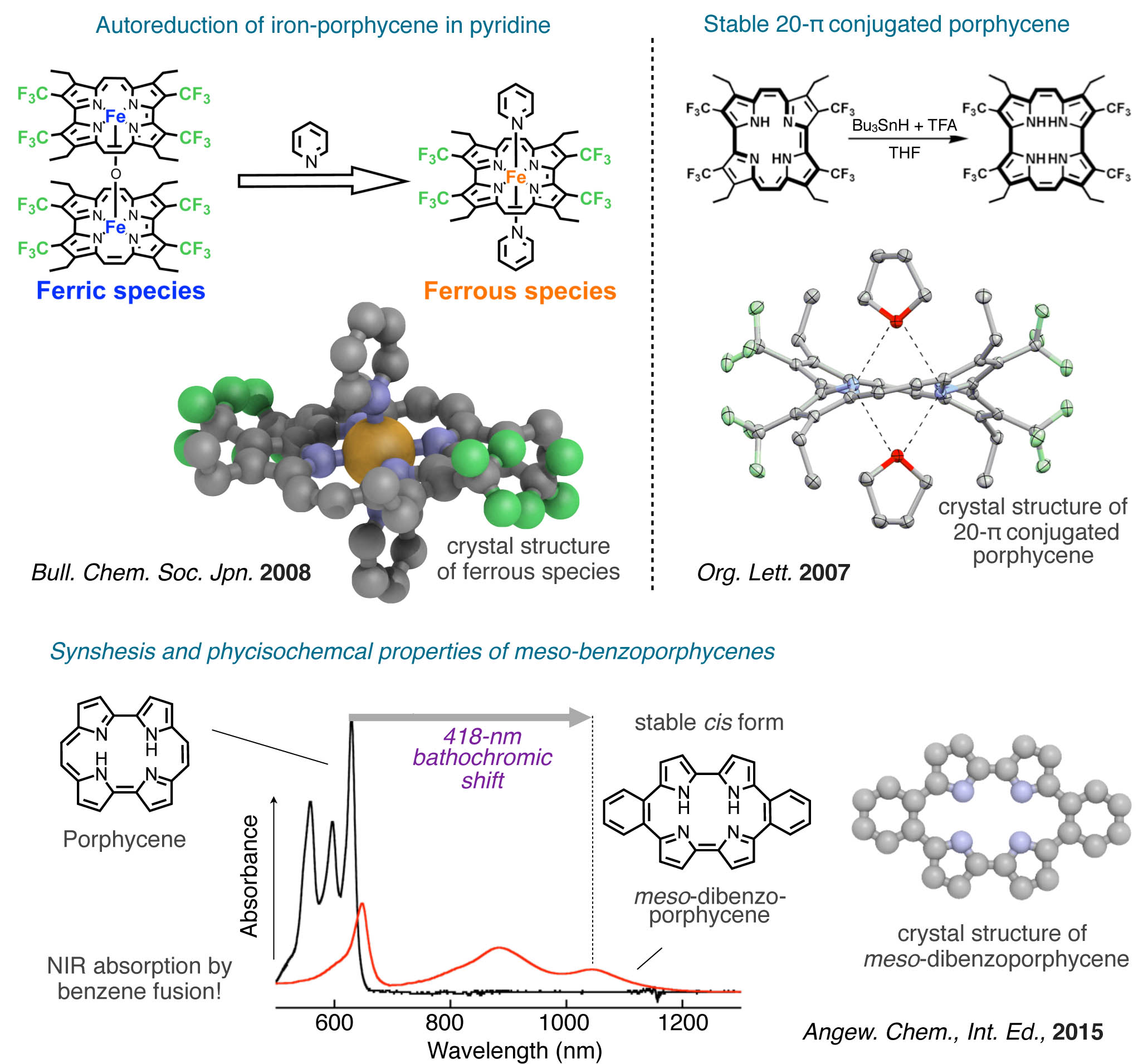
- 39) Inorg. Chem., 50, 4892–4899 (2011).
- 40) Inorg. Chem., 52, 13014–13020 (2013).
- 41) Chem Commun., 50, 3421–3423 (2014). Front Cover.
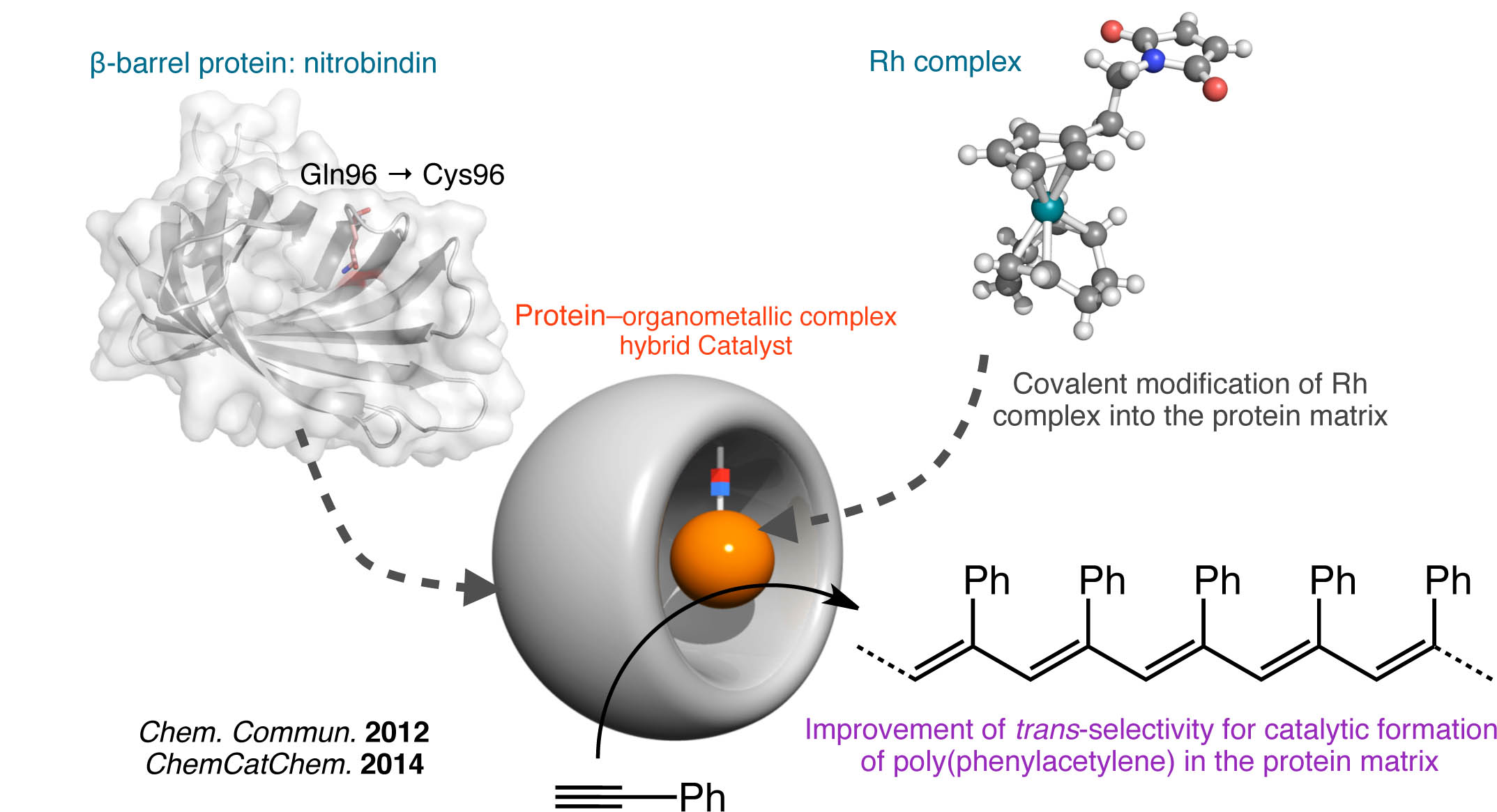
Hybrid Biocatalyst using Organometallic Complex and Protein Matrix
In biological systems, various native metalloenzymes have acquired the ability to precisely control reactivity of the metal center and specificity for substrates by interactions provided by protein matrices. Inspired by these natural systems, we envision creation of artificial metalloenzymes catalyzing unnatural reaction, which is an attractive subject for development of novel catalysts. As the active sites for such catalyst, our group has focused on organometallic complexes that can be incorporated within protein matrices. In our reported example, we constructed a hybrid biocatalyst containing a Rh complex immobilized within the cavity of the β-barrel protein, which archived improved trans-selectivity in polymerization of phenylacetylene.42,43
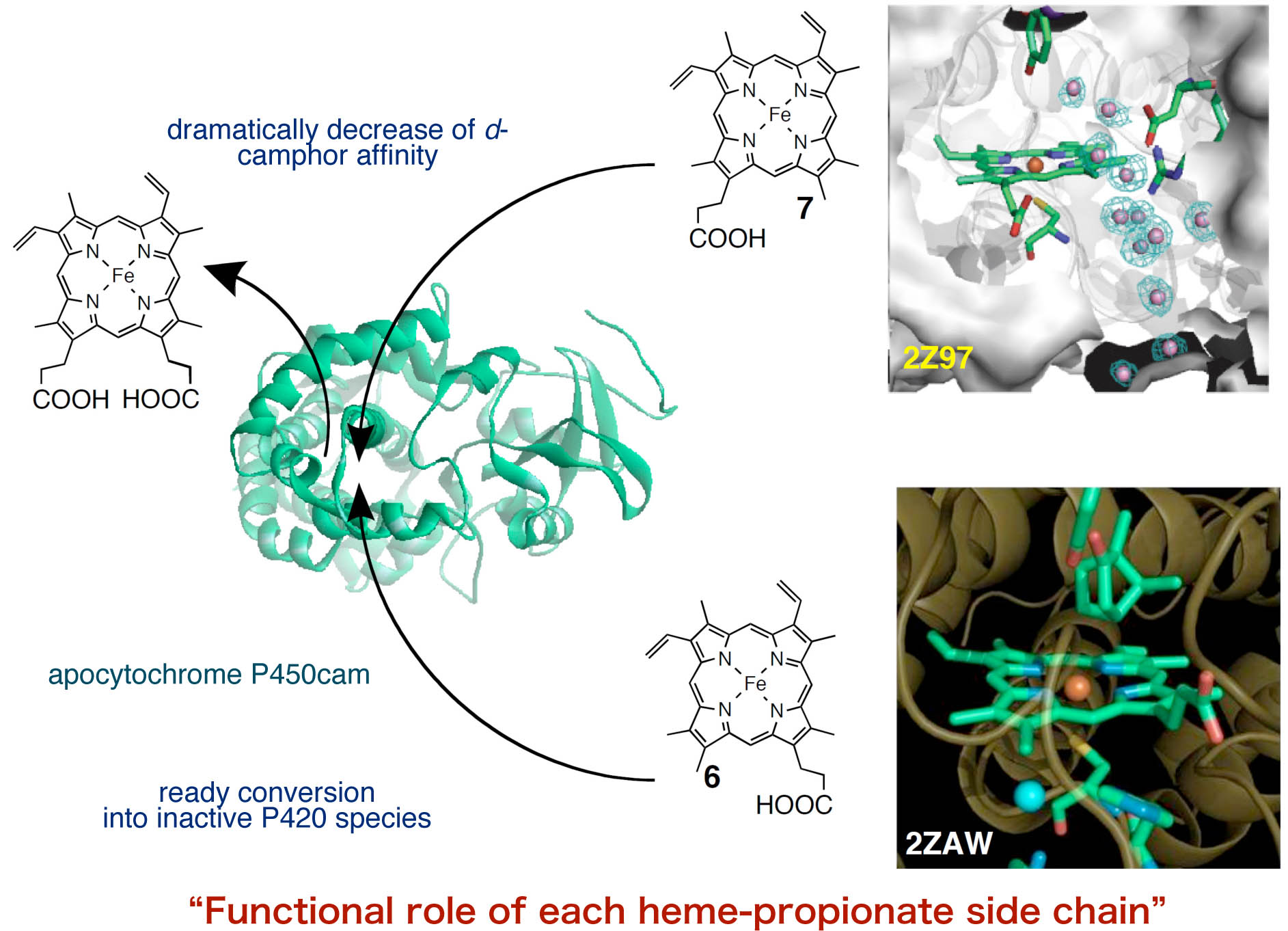
Functional Role of Heme-Propionate Side Chain in Hemoprotein
Heme b has two propionate side chains. It has been believed that the propionate side chains play a role on the anchor of the prosthetic group heme to bind with hemoprotein interior. We propose the functional significance of those propionate side chains by reconstituted myoglobins with two kinds of one-legged hemes where one of the propionates is replaced with a methyl group. In sperm whale myoglobin, it is found that the heme-6-propionate side chain stabilizes the bound dioxygen via a unique hydrogen bonging network in the distal pocket.44 In monooxygenase cytochrome P450cam, the heme-6-propionate side chain is essential for the maintenance of the enzyme activity, because the removal of the side chain converts the active enzyme into an inactive P420 species, although the crystal structure of the reconstituted protein is almost same as that of the wild type protein.45 The removal of the heme-7-propionate side chain dramatically decreases the substrate d-camphor affinity and decelerates the catalytic hydroxylation of d-camphor.46
New Porphyrinoid Chemistry
Tetrapyrrolic metalloporphyrin derivatives have been widely studied as biomimetic models for hemoproteins. They also have a potential to act as an attractive catalyst in organic and coordination chemistry. We have focused on an attractive porphyrin isomer, particularly porphycene, with reduced symmetry, because the LUMO of the 18π-macrocyclic porphycene is remarkably stabilized compared to the corresponding porphyrin. We prepared metalloporphycene having strongly electron-withdrawing trifluoromethyl groups at the pyrrole β-positions to obtain an extremely electron-deficient tetrapyrrole macrocycle.47 The unusual characteristics of porphycene and metalloporphycene species provide a stable low-valent metal complex48,,49 an interelement complex containing an M–M bond50 and a 20π-nonaromatic porphycene51,52 and unusual cis form in benzene-fused porphycene at meso positions.53 Furthermore, we started to investigate the unique reactivity of a cobalt corrole complex with ethyl diazoacetate and properties of obtained characteristic N-bridged product.54
- 47) Org. Lett., 5, 2845–2848 (2003).
- 48) Inorg. Chem., 42, 7345–7347 (2003).
- 49) Bull. Chem. Soc. Jpn., 81, 76–83 (2008). BCSJ Award
- 50) J. Porphyrins Phthalocyanines, 16, 616–625 (2012).
- 51) Org. Lett., 9, 5303–5306 (2007).
- 52) J. Org. Chem., 77, 8946–8955 (2012).
- 53) Angew. Chem., Int. Ed., in press (2015).
- 54) Organometallics, 30, 1869–1873 (2011).